Did you know that the Food and Drug Administration (FDA) has specific labeling requirements for cosmetics? If you want to make sure your products are compliant, read on. This blog post will cover the basics of FDA cosmetics labeling requirements, including information on ingredients, net weight, and directions for use. By understanding these guidelines, you can create labels that meet FDA standards and help ensure your products are safe and effective. Let’s get started!
FDA cosmetic labeling requirements
The FDA has specific labeling requirements for cosmetics, which must be followed in order to sell your products legally in the United States. These requirements are designed to ensure that consumers have the information they need to use cosmetics safely and effectively.
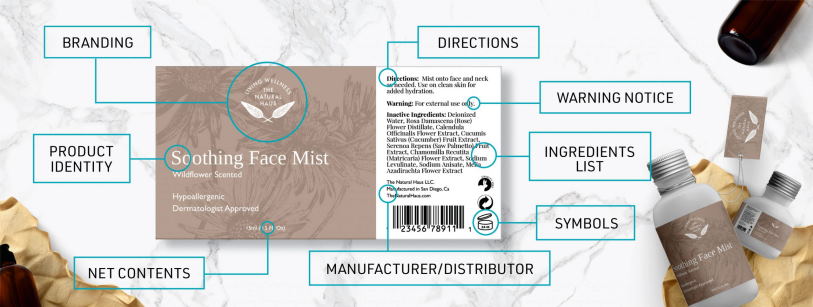
In general, all cosmetics must be labeled with the following information:
- The product name
- The identity of the manufacturer or distributor
- The net quantity of contents
- The directions for use, if needed
- Any warnings required by law or regulation
In addition, certain types of cosmetics must also include an ingredient list on the label. This is generally required for products that are intended to be left on the skin for a prolonged period of time, such as lotions and creams.
The FDA also has regulations concerning the claims that can be made on cosmetic labels. These claims must be truthful and not misleading, and they must be supported by scientific evidence. For example, a claim that a particular cream will “cure” wrinkles would likely be considered misleading unless it is supported by scientific studies.
If you are selling cosmetics in the United States, it is important to familiarize yourself with the FDA’s labeling requirements. Failure to comply with these requirements can result in legal penalties, including fines and product recalls.

Issues in the safety and labeling of cosmetics products
The safety and labeling of cosmetics products have come under increased scrutiny in recent years. A number of studies have raised concerns about the potential health risks posed by certain ingredients in these products, as well as the lack of clear and accurate labeling on many cosmetics products.
One major concern is the use of synthetic chemicals in cosmetics products. Many of these chemicals have not been properly tested for safety, and there is growing evidence that they may pose serious health risks. For example, some synthetic chemicals used in cosmetics have been linked to cancer, birth defects, and other serious health problems.
Another concern is the lack of clear and accurate labeling on many cosmetics products. Many products do not list all of their ingredients, or they may use misleading terms such as “fragrance” or “paraben-free.” This makes it difficult for consumers to know what they are buying and to make informed decisions about which products are safe to use.
The safety and labeling of cosmetics products is an important issue that needs to be addressed. Consumers should be aware of the potential risks posed by these products and should carefully read labels before purchase.